Periprosthetic Infection Following Total Elbow Arthroplasty: A Case Report and Review of the Literature
Monica M. Shoji, MD, Stella J. Lee, MD, George S.M. Dyer, MD, FACS
The authors report no conflict of interest related to this work.
©2019 by The Orthopaedic Journal at Harvard Medical School
Total elbow arthroplasty can provide pain relief and functional improvement in inflammatory arthritis and osteoarthritis of the elbow, as well as acute complex distal humerus fractures in the elderly, but periprosthetic infections of the elbow are difficult to treat. This article aims to provide a comprehensive review of diagnosis and treatment options for periprosthetic infections following total elbow arthroplasty. The mainstay of treatment consists of implant removal, thorough debridement with complete synovectomy, placement of an antibiotic spacer, and intravenous antibiotics for 6 weeks, followed by reimplantation after clinical and laboratory evidence of infection eradication. The creation of a functional antibiotic spacer provides some stability and range of motion and even allows for definitive treatment with a spacer depending on medical comorbidities and functional goals of the patient.
LEVEL OF EVIDENCE Level V Narrative Review
KEYWORDSTotal elbow replacement, total elbow arthroplasty, periprosthetic joint infection
Total elbow arthroplasty (TEA) has become increasingly common over the past decade, with the number of TEAs performed in the United States doubling from 1998 to 2011.1 Indications include inflammatory arthropathies (such as rheumatoid arthritis), post-traumatic arthritis, and acute complex distal humerus fractures in elderly patients.2 While TEA can provide pain relief and functional improvement in appropriately selected patients, the risk of complications and subsequent revision surgery associated with TEA remains high. This is particularly true when TEA is used in the setting of acute trauma where a re-operation rate of greater than 50% within 6 years has been reported.3
Periprosthetic joint infection (PJI) rates for TEA range between 1.5% and 12% nationally.4-6 Approaches to treatment of periprosthetic elbow infections have been modified from protocols for hip and knee infections, and options vary from suppressive antibiotics to revision procedures including irrigation and debridement with implant retention, revision arthroplasty, and resection arthroplasty. This article aims to present a case of periprosthetic infection of a total elbow arthroplasty and to provide a comprehensive review of diagnosis and treatment options for periprosthetic infection following total elbow arthroplasty.
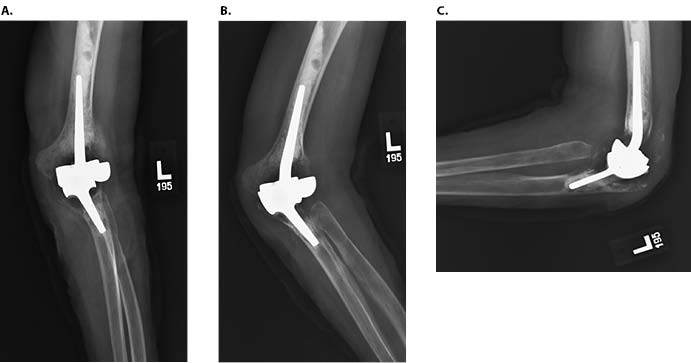
A 71-year-old woman underwent a left total elbow arthroplasty fifteen years prior to presentation for end-stage rheumatoid arthritis that failed non-operative management. She did well for fourteen years, but over the course of ten weeks, she had increasing swelling, pain, and subjective instability accompanied by erythema. A purulent draining sinus tract overlying the olecranon ultimately developed. On examination, she had passive range of motion from -10° of extension to 120° of flexion. She reported pain with varus and valgus stress but did not demonstrate frank instability. No neurologic deficits were appreciated distally. Aspiration of synovial fluid from the elbow revealed 2090 nucleated cells with 88% neutrophils. Cultures were negative. Radiographs showed significant osteolysis (Figure 1) and complete mechanical failure of the elbow.
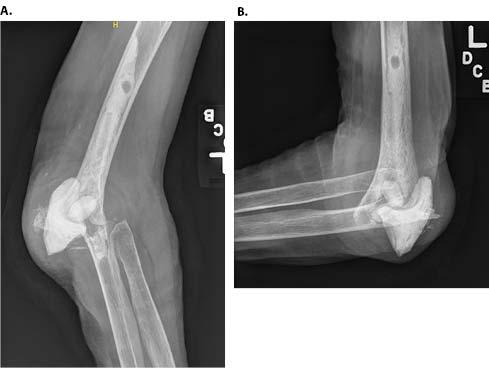
A diagnosis of periprosthetic joint infection was made, and the patient was treated with staged revision. Intraoperatively, it was noted that the elbow implant had extruded directly through the extensor mechanism. The implants were easily removed, the reactive osteolysis membrane was debrided, and the sinus tract was excised. Tissue from about the elbow was sent intraoperatively to microbiology for culture prior to the administration of antibiotics. The wound was irrigated, and an antibiotic spacer was fashioned (Figure 2). The intraoperative cultures grew methicillin-sensitive Staphylococcus aureus. Consultation from the infectious diseases service was sought, and the patient was treated with four weeks of intravenous vancomycin. Ten weeks after completion of intravenous antibiotic therapy, she had no clinical signs of infection, and re-aspiration of synovial fluid from the elbow yielded no growth. She underwent second-stage surgery with removal of the functional antibiotic spacer and reimplantation of a revision TEA with antibiotic cement (Figure 3).
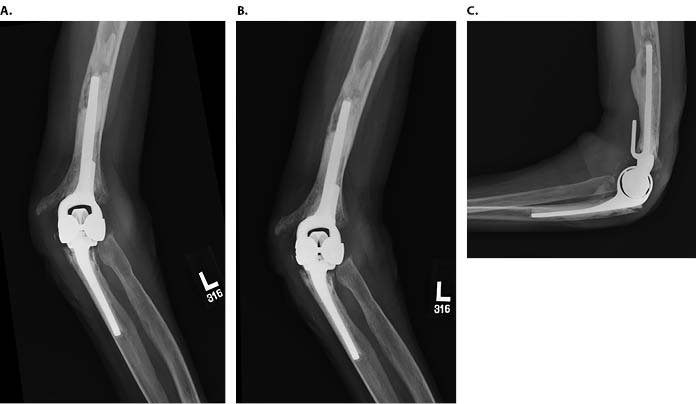
Five weeks following reimplantation, the patient developed increasing pain and drainage and underwent urgent irrigation and debridement with an extensive synovectomy and revision of the polyethylene bearing surface. Intraoperative cultures again grew Staphylococcus aureus, and the patient was treated with six weeks of intravenous nafcillin followed by three months of levofloxacin and rifampin, per the guidance of the infectious diseases service. Six months later, she developed recurrence of a draining sinus tract indicative of recurrence of periprosthetic joint infection.
Infection After TEA
Risk factors for periprosthetic joint infect (PJI) include previous elbow surgery, history of previous elbow infection, psychiatric illness, severe rheumatoid arthritis, post-operative wound drainage, and re-operation for any reason.7,8 Evaluation for PJI is warranted with a history of increasing elbow pain, progressive loss of elbow function, erythema, and warmth about the elbow; suspicion for PJI is further heightened with an elevated ESR or CRP.9-12
The Musculoskeletal Infection Society (MSIS) published guidelines in 2011 for diagnosis of prosthetic joint infection in total hip arthroplasty and total knee arthroplasty.13 While there are no universally accepted guidelines for defining infection in total elbow arthroplasty, the British Elbow and Shoulder Society recommends utilizing the MSIS criteria for diagnosis of periprosthetic elbow infections.14 The MSIS criteria for a prosthetic joint infection (PJI) include a sinus tract communicating with the prosthesis, a pathogen present in at least two tissue or fluid samples from the prosthetic joint, and/or a combination of multiple elevated inflammatory markers.13
To obtain optimal data about the pathogen, antibiotics are discontinued for several days prior to revision arthroplasty, and perioperative antibiotics are held prior to obtaining tissue samples for culture. The most common causative organisms include Staphylococcus epidermidis and Staphylococcus aureus.7,10 Less commonly, Escherichia coli, ß-hemolytic Streptococcus species, and fungal pathogens are implicated.8-11,15
Treatment Options
For confirmed cases of periprosthetic joint infection, the most common treatment options are one- versus two-stage revision arthroplasty and irrigation and debridement with retention of implants. Other treatments that have been attempted include suppressive antibiotics, resection arthroplasty, and resection arthrodesis.
Two-Stage Revision Arthroplasty
A two-stage revision involves complete removal of the prosthesis, often with placement of an antibiotic-laden cement spacer, followed by the administration of pathogen-targeted antimicrobial therapy and subsequent placement of a new prosthesis once the infection has been eradicated. A two-stage revision arthroplasty may help to restore function; however there is a paucity of data reporting risk of recurrent infection or rates of failure. Peach et al. reported a case series of staged revision surgery for the treatment of 34 infected TEAs in 33 patients who underwent a first stage procedure involving the insertion of antibiotic-impregnated cement beads with only 24 hours of perioperative cefuroxime.16 No additional oral or intravenous antibiotic therapy was administered prior to second-stage re-implantation. In these patients, 15% required at least one additional irrigation and debridement prior to proceeding to the second stage reimplantation of hardware. Recurrent infections occurred in 11.5% of patients after the second-stage procedure.16
In contrast, Rudge et al. recommended reimplantation of hardware only after an initial first-stage revision with removal of hardware, irrigation and debridement, and insertion of an antibiotic-loaded cement spacer, followed by at least six weeks of intravenous antibiotics.7 Two weeks after cessation of antibiotics, a fluoroscopic-guided aspiration of the affected joint was performed to assess for persistent infection. If the aspirate was negative, then a second-stage procedure was considered; none of their 14 patients required repeat debridement after the second stage procedure.7
The mean Mayo Elbow Performance Score (MEPS) in patients who underwent a second-stage revision without recurrence of infection was 81.1 (range of 65 to 95).7 The mean MEPS in patients with recurrence of infection was 50 (range of 30 to 70).7 Cheung et al. reported that in patients who underwent two-stage revision due to infection, further operations were performed for recurrent infection in 52% of patients at 8 years.10
One-Stage Revision Arthroplasty
A one-stage revision arthroplasty involves complete removal of the prosthesis, thorough irrigation and debridement of the joint, and placement of a new prosthesis in a single procedure. Proposed advantages of a one-stage revision include decreased morbidity, reduced cost, and preservation of a well-functioning joint.9 However, if the infection is not eradicated, additional procedures may be necessary. In a series of six patients, two out of six had recurrent infections, one of whom required removal of the prosthesis. The four remaining patients were satisfied with their outcome.9 Further studies are needed to establish the indications for a one-stage revision.
Irrigation and Debridement with Retention of Implants
Several variables must be considered when attempting to retain implants during the treatment of an infected TEA, including duration of infection, causative pathogen, medical status of the patient, and implant stability.6,8,11 Irrigation and debridement without component removal can succeed in certain cases but only if the components are well-fixed.6,8 Wolfe et al. examined outcomes of multiple irrigation and debridement procedures in combination with suppressive antibiotics.8 Eight of the ten patients had persistent infection, ultimately resulting in resection arthroplasty in six patients and arthrodesis in two patients.8
Yamaguchi et al. reported in their case series that all four Staphylococcus epidermidis infections recurred despite extensive irrigation and debridement involving complete disarticulation of the components, removal of bushings, and placement of tobramycin-impregnated cement beads with concurrent targeted intravenous antibiotic therapy.6 The ability of S. epidermidis to form effective biofilm is thought to contribute to its persistence thereby necessitating implant removal for eradication of infection.6
Antibiotic Suppression
Antibiotic therapy alone without debridement or implant removal is reserved for severely ill patients who are unable or unwilling to tolerate surgery. With this approach, the treatment of periprosthetic infections has a high failure rate.8,11,12,17 Eradication of deep infection necessitates surgical intervention.
Resection Arthroplasty
Resection arthroplasty is generally reserved for those patients with very low functional demand or very high surgical risk because patients are frequently left with significant functional deficits. The clinical outcome after excision arthroplasty depends on the ability to preserve the medial and lateral condyles of the distal humerus to provide a stable fulcrum for the elbow joint.10,11 Removal of the cement and implant can be technically difficult and result in marked bone loss that can lead to gross instability, a shortened extremity with decreased effective muscle strength, and a flail elbow.8,10 Wolfe et al. reported that three of eight patients who underwent resection arthroplasty sustained a fracture during the procedure.8 Morrey et al. found that results of resection arthroplasty after infection—assessing for pain, motion, and stability—were “good” in six patients and “fair” in five, with failure in one due to painful ankylosis.11
Resection Arthrodesis
Arthrodesis of the elbow is rarely performed as the resultant loss of functional motion can also be debilitating. Wolfe et al. reported on two patients who failed multiple irrigation and debridement procedures and ultimately underwent arthrodesis with the elbow in 90° of flexion.8 Both patient outcomes were classified as failures: one developed a fibrous ankylosis with a relatively painless but functionally limited extremity, and the other had persistent pain and wound drainage despite a second attempt at fusion.
Author's Technique
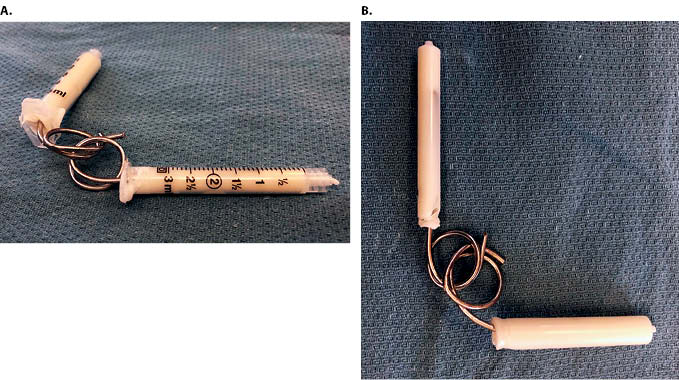
The senior author’s treatment of choice for periprosthetic infection of the elbow is a two-stage exchange. The first-stage procedure consists of a complete implant resection with thorough irrigation and debridement of all contaminated synovium, devitalized tissue, sinus tract, and any remaining cement. All areas of the wound are cleansed with 0.5% Dakin’s (dilute sodium hypochlorite) solution and then irrigated with six liters of normal saline. A functional linked antibiotic spacer is created by using two 2.4 mm K-wires, which are bent by hand using vice grips, to create two interlocking sets of rings. The cement spacers are sized to fit proximally in the humerus and distally in the ulna; generally two 3 mL syringes are adequate (Figure 4). Once dry, the spacers should fit easily within the intramedullary canals and be removable after the first stage. The wires are “potted” in antibiotic-laden bone cement to pad the sharp tips of the wires and to provide local antibiotic delivery. Either Palacos® with gentamycin (Zimmer Biomet, Warsaw, Indiana, USA) or Simplex® with tobramycin (Stryker Corporation, Kalamazoo, Michigan, USA) are used. The spacer should allow full elbow extension without hyperextension. Although intended for temporary use, this linked spacer may also be used as definitive treatment in select cases. This first stage is followed by six weeks of intravenous antibiotics tailored to intra-operative culture data, followed by an additional six weeks of observation off antibiotics. An elbow aspiration is repeated at that time to determine suitability for revision arthroplasty.
Periprosthetic elbow infections are challenging. The most reliable treatment results have been seen with a two-stage revision, although outcomes data are limited. The creation of an antibiotic spacer that functions as a hinge may provide some stability and range of motion and may allow for definitive treatment with a spacer depending on the functional goals of the patient. Unfortunately, as seen in this case, infections can recur even after a staged exchange. Further studies are needed to determine the risk factors for treatment failure and to help develop evidence-based guidelines for management of periprosthetic infections specific to the elbow.






