Mass Spectrometry For Glycoform Analysis of Glycosaminoglycans: A New Approach to an Old Problem
Karen E. Yates, PhD, Joseph Zaia, PhD
Brigham and Women’s Hospital
 Introduction
Introduction
Interplay between protein and carbohydrate components
in the extracellular matrix sustains the unique biomechanical
properties of cartilage. Long-chain sugars known as glycosaminoglycans
(GAGs) are enmeshed within a collagen lattice. Water
is attracted to the highly-6sulfated GAG chains and causes
them to swell. Because GAGs are constrained within a collagen
lattice, the swelling generates hydrostatic pressure that enables
cartilage to bear loads. Loss of GAGs from the matrix, as well
as changes in composition or sulfation, may set the stage for
degeneration of cartilage tissue.
Technical constraints of current analytical methods limit
our knowledge of GAG structures (glycoforms) in normal cartilage
and the changes that occur with pathologic conditions.
Most methods require complete digestion of GAGs into disaccharides
prior to analysis. The abundance of different disaccharide
forms can be measured, but information on their arrangement
(patterning) is lost. New approaches are needed to obtain
quantitative information on patterning and other structural
variables. The objective of this interdisciplinary collaboration is
to adapt novel, mass spectrometry-based platform technologies
to analyze the fine structure of GAGs from human cartilage.
GLYCOSAMINOGLYCAN STRUCTURE
Proteoglycans are comprised of one or more GAGs attached
to a protein core (Figure 1). Subtypes of GAGs such as keratan
sulfate (KS) or chondroitin sulfate (CS) contain characteristic
combinations of disaccharide units. Additional structural diversity
in GAGs is generated by sulfate modification and epimerization
of uronic acid within the disaccharides. Chondroitin sulfate
and dermatan sulfate are closely related in structure; dermatan
sulfate is distinguished by having disaccharide repeats that
contain iduronic acid, rather than glucuronic acid. The components
of disaccharides may be sulfated at different locations,
such as the 4 postion in galactosamine (chondroitin 4-sulfate,
or chondroitin sulfate type A) or at the 6 position (chondroitin
6-sulfate, or chondrotin sulfate type C).

Changes in cartilage GAG composition and sulfation occur
during normal development and with disease. For example,
the number of KS chains on aggrecan increases after the age
of ~20 years 1,2, and there is a shift in the relative amounts of
chondroitin 4-sulfate and chondroitin 6-sulfate 3-5. Changes
that occur in osteoarthritic (OA) cartilage may be distinct from
aging, such as a decrease in KS 6 and altered sulfation at terminal
residues 7. With current analytical methods that require
complete digestion of GAGs into disaccharides, much of the
information on structural variables is destroyed. That information
could be retained, however, by using methods that are able
to distinguish larger oligosaccharides (Figure 2).
A MASS SPECTRAL APPROACH TO ANALYZE GLYCOSAMINOGLYCAN STRUCTURE
In mass spectral analysis, different chemical structures
display characteristic peaks of ion abundance (i.e., diagnostic
ions). Specific structures can be identified and quantified by
measuring the abundance of diagnostic ions. Several types of
mass spectral approaches have been developed for analysis of
biomolecules, including proteins and DNA.
Dr. Zaia’s group at the Mass Spectrometry Resource, Boston
University School of Medicine proposed that a combination of
liquid chromatography (LC) and tandem mass spectral analysis
(MS/MS) could be used for direct analysis of oligosaccharides,
to obtain patterning information without additional purification
and digestion steps 8,9. The determination of chondroitin
sulfate sulfation using disaccharide analysis is a well-established
method. Determination of dermatan sulfate content, however,
requires multiple steps separate from disaccharide analysis.
Dr. Zaia’s group has shown that analysis of tetramer oligosaccharides
of cartilage CS using mass spectrometry determines
sulfation and epimerization simultaneously. This approach was
validated through a series of experiments measuring CS glycoforms
in dermatan sulfates from porcine skin, decorins from
three different tissues (articular cartilage, sclera, and cervix),
and cartilage extracts 8,10. The next step was to apply this novel
methodology to GAGs from tissue samples.
APPLICATION OF THE MASS SPECTRAL APPROACH TO CARTILAGE TISSUE
The biology of cartilage presents technical challenges
for analysis of GAGs by any method. Regional variations in
the tissue (especially with degeneration and disease) impede
characterization within subtle or focal alterations. Analysis by
LC-MS/MS is additionally challenging because of the amount
and purity of starting material required. Articular cartilage
excised from the shoulder joint of a juvenile calf was used in
a series of experiments to begin addressing
these issues.

Initially, we attempted to use standard
procedures to extract GAGs from intact tissue
for mass spectral analysis. Cartilage was
digested with papain and CS oligosaccharides
were prepared by standard methods.
Mass spectra of those samples, however,
showed a high level of noise, and the CS
diagnostic ion peak at 458 m/z was not discernable.
In a series of optimization experiments
11, we successfully developed a modified
sample workup procedure that produces
high-quality analytical data on GAGs from
cartilage tissue (Figure 3). Glycoform abundances
in CS were then measured with that
optimized sample workup. In a sample of
articular cartilage from a young calf, 57.9%
of tetrasaccharides were chondroitin 4-sulfate-like, 40.1% were
chondroitin 6-sulfate-like, and 2% were dermatan sulfate-like.
To validate those results, capillary electrophoresis (CE) was
used to measure disaccharides in the same sample. The measured
amounts of 4-sulfated (57.5%), 6-sulfated (39.1%) and
unsulfated disaccharides (3.4%) were in good agreement with
the glycoform data obtained by LC-MS/MS.
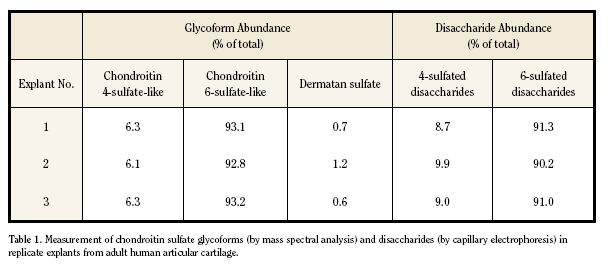
GLYCOFORM ANALYSIS OF HUMAN CARTILAGE
Reproducibility of glycoform measurements with the
LC-MS/MS platform was evaluated with human articular cartilage
samples. Three explants of normal-appearing tissue were
excised from a tibial plateau that was discarded during total
knee arthroplasty for osteoarthritis. Triplicate aliquots of each
papain-digested explant were subjected to the optimized sample
workup procedure for mass spectral analysis. Measurement
of CS glycoforms was highly reproducible and again showed
good agreement with disaccharide compositions measured
by CE (Table 1). The low abundance of chondroitin 4-sulfate
glycoforms measured in those samples was consistent with
published data for adult cartilage.
A larger set of samples obtained from 5 donors
(13 explants, weighing 16-60 mg) was analyzed to
determine the variance of glycoform measurements.
In that group of samples, the mean abundance of
chondroitin 6-sulfate-like tetrasaccharides was 90.4%
± 3.2 and chondroitin 4-sulfate-like was 8.5% ± 3.1.
Those quantities were similar to our other results
with adult cartilage. Variance between triplicate aliquots
that were analyzed for each sample was very
low for chondroitin 6-sulfate-like tetrasaccharides
(coefficient of variation = 1.9%). For chondroitin
4-sulfate like tetrasaccharides, variance was greater
(21.5%) and was likely due to the low abundance
of that glycoform. Nonetheless, these results show
that the LC-MS/MS approach is a sensitive, accurate
method to quantify glycoform structures from as little
as 16 mg of cartilage tissue.
THE NEXT STEPS
This work demonstrates the feasibility of mass spectral
approaches for glycoform analysis of GAGs. At this stage, the
methodology is highly sensitive and requires just 10 µg of GAGs
from each sample. That threshold is expected to decrease as
more powerful instrumentation is developed. Another benefit
that will come with enhanced sensitivity is the potential to
quantify rare structures that are not detectable by other means.
Ultimately, the goal is to develop a true “glycomic” approach
for simultaneous analysis of multiple GAGs. These methods will
uncover new insights into the structure-function relationships
of GAGs in cartilage.
Acknowledgements. The authors thank Ms. Alicia
Hitchcock (BUSM) and Dr. Sonya Shortkroff (BWH) for their
participation in these studies, and Drs. Thomas Thornhill and
John Wright (BWH) for providing human cartilage samples.
This work was supported by NIH grants AG023307 (KEY),
HL74197 (JZ), and the BUSM Mass Spectrometry Resource for
Biology and Medicine (P41 RR10888).
Karen E. Yates, PhD, Instructor in Orthopedic Surgery, Brigham and Women’s Hospital and Harvard Medical School.
Joseph Zaia, PhD, Associate Research Professor of Biochemistry and Associate Director, Mass Spectrometry Resource, Boston University School of Medicine.
Address correspondence to:
Karen E. Yates, Ph.D.
Orthopedic Research
Brigham and Women’s Hospital
75 Francis Street
Boston, MA 02115
References:
- Maroudas A, Bayliss MT, Venn MF. Further studies on the composition of human femoral head cartilage. Ann Rheum Dis 1980;39(5):514-23.
- Venn MF. Variation of chemical composition with age in human femoral head cartilage. Ann Rheum Dis 1978;37(2):168-74.
- Cheng F, Heinegard D, Fransson L, Bayliss M, Bielicki J, Hopwood J, Yoshida K. Variations in the chondroitin sulfate-protein linkage region of aggrecans from bovine nasal and human articular cartilages. J Biol Chem 1996;271(45):28572-80.
- Lauder RM, Huckerby TN, Brown GM, Bayliss MT, Nieduszynski IA. Age-related changes in the sulphation of the chondroitin sulphate linkage region from human articular cartilage aggrecan. Biochem J 2001;358(Pt 2):523-8.
- Plaas AH, Wong-Palms S, Roughley PJ, Midura RJ, Hascall VC. Chemical and immunological assay of the nonreducing terminal residues of chondroitin sulfate from human aggrecan. J Biol Chem 1997;272(33):20603-10.
- Aigner T, Hemmel M, Neureiter D, Gebhard PM, Zeiler G, Kirchner T, McKenna L. Apoptotic cell death is not a widespread phenomenon in normal aging and osteo arthritis human articular knee cartilage: a study of proliferation, programmed cell death (apoptosis), and viability of chondrocytes in normal and osteoarthritic human knee cartilage. Arthritis Rheum 2001;44(6):1304-12.
- Plaas AH, West LA, Wong-Palms S, Nelson FR. Glycosaminoglycan sulfation in human osteoarthritis. Disease-related alterations at the non-reducing termini of chondroitin and dermatan sulfate. J Biol Chem 1998;273(20):12642-9.
- Hitchcock AM, Costello CE, Zaia J. Glycoform quantification of chondroitin/dermatan sulfate using a liquid chromatography-tandem mass spectrometry platform. Biochemistry 2006;45(7):2350-61.
- Zaia J, Li XQ, Chan SY, Costello CE. Tandem mass spectrometric strategies for determination of sulfation positions and uronic acid epimerization in chondroitin sulfate oligosaccharides. J Am Soc Mass Spectrom 2003;14(11):1270-81.
- Miller MJ, Costello CE, Malmstrom A, Zaia J. A tandem mass spectrometric approach to determination of chondroitin/dermatan sulfate oligosaccharide glycoforms. Glycobiology 2006.
- Hitchcock AM, Shortkroff S, Yates KE, Costello CE, Zaia J. Optimized extraction of glycosaminoglycans from cartilage for disease state glycomics.; 2006; Seattle, WA.
|