Vertebral Collapse and Cord Compression in a Young Adult With Paget’s Disease of the Spine
Darren Lebl, MD and Paul Glazer, MD
Beth Israel Deaconess Medical Center
HISTORY AND PHYSICAL EXAM
 A 38-year-old Caucasian male presented to our institution
with a four year history of intermittent low back pain. There
was no trauma or inciting event and his pain progressed over
the course of months to radiate to both of his lower extremities.
Initially he was self-treated with conservative measures
such as physical therapy, ergonomic chairs, and massages.
He was capable of going to work, playing golf, working out at
the gym, and was able to sit for prolonged periods of time at
work with ibuprofen. His associated symptoms included a ten
pound weight loss over the preceding months and a progressive
kyphosis. He denied fevers, chills, night sweats, headache,
visual/auditory changes, bowel or bladder dysfunction and
fatigue. History was negative for environmental exposures
(radiation, asbestos, etc).
A 38-year-old Caucasian male presented to our institution
with a four year history of intermittent low back pain. There
was no trauma or inciting event and his pain progressed over
the course of months to radiate to both of his lower extremities.
Initially he was self-treated with conservative measures
such as physical therapy, ergonomic chairs, and massages.
He was capable of going to work, playing golf, working out at
the gym, and was able to sit for prolonged periods of time at
work with ibuprofen. His associated symptoms included a ten
pound weight loss over the preceding months and a progressive
kyphosis. He denied fevers, chills, night sweats, headache,
visual/auditory changes, bowel or bladder dysfunction and
fatigue. History was negative for environmental exposures
(radiation, asbestos, etc).
On examination, his thoracolumbar junction was nontender
and kyphotic with a compensatory increase in lumbar
lordosis. There was no atrophy of the lower extremities and no
sensory deficits in the lower extremities. Dorsalis pedis pulses
were palpable and equal bilaterally. Babinski’s reflex was absent
on the right and upgoing on the left. There were hyperreflexive
patellar and Achilles reflexes, no clonus, and a negative straight
leg raise.
Initial work-up included plain radiographs which revealed
a destructive process at T12 and L1 with severe compression
deformity and loss of vertebral height. There was 30 degrees
of kyphosis on the lateral radiograph. A grade I listhesis to
the right at the thoracolumbar junction and retropulsion of
fracture fragments into the spinal canal were also visualized.
CT scan showed pedicle destruction and eccentric soap bubble
appearance of the involved levels with posterior element sparing
(Fig. 1).
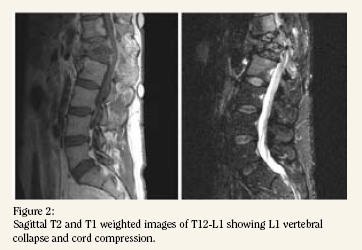
An MRI with gadolinium was obtained which confirmed
a destructive process involving T12-L1 causing spinal stenosis
and cord compression from T11 to L2 and involving the associated
paraspinal soft tissues (Fig. 2). A CT guided transpedicular
biopsy was performed of T12 which showed fragments of woven
bone with reactive changes and was negative for malignant
cells. A joint fluid sample was obtained from the T12/L1 disc
space which was negative on gram stain, fluid culture, and acid
fast smear. His alkaline phosphatase was elevated (249 IU/L).
HOSPITAL COURSE
The patient was taken to the operating room for vertebrectomy
of T12-L1, anterior cage replacement of T12 and L1
and fusion with rib autograft. Intra-operatively, a large soft tissue
mass was visualized covering the spine on the left side of
the vertebral body which appeared to be bony callus. This was
resected and sent to pathology. There was severe collapse of
the T12 and L1 vertebral bodies with bone and disc retropulsed
against the conus. The fractured vertebrae were gently elevated
off the spinal cord, resected, and a thorough decompression
was performed. There were also extensive adhesions to the
posterior longitudinal ligament by calcified pagetic tissue. On
post-operative day #2 he was taken back to the OR for posterior
fusion from T10 to L3 for his kyphotic deformity. Post-operatively
the patient reported neurological improvement.
The histopathological diagnosis revealed characteristic
“mosaic” pattern of disordered bone formation and osteoclastlike
giant multi-nucleated cells characteristic of Paget’s disease
(Fig. 3).
DISCUSSION
Osteitis deformans or Paget’s disease of bone (PD) is a
common disorder of abnormal bone homeostasis in the elderly
population. PD is generally diagnosed by exam, serum alkaline
phosphatase (AP) levels, plain radiographs, and bone scintigraphy
and treated medically1. The prevalence of PD increases
with age and few reports exist in the literature of severe spinal
deformity secondary to PD in persons under age 40. Here we
report a case of focal PD at the thoracolumbar junction causing
progressive deformity, mechanical overload of the T12 and
L1 vertebral bodies and cord compression. PD uncommonly
presents as pathological vertebral compression fracture causing
myeloradiculopathy and requiring acute decompression.
Despite the high prevalence of PD in the geriatric population,
isolated and symptomatic pagetic lesions of the spine are rare
to occur in young adult patients.
PD is the second most common disorder affecting bone
after osteoporosis2 and the lumbar (58%), thoracic (45%), and
cervical (14%) spines are frequently involved3. The overall
prevalence of PD in the United States has been estimated to be
between 1 and 2% or approximately 2.5-5 million people. The
adult onset form of PD (distinct from juvenile PD) increases
in prevalence with age and rarely becomes symptomatic in a
young healthy adult4.
Maintenance of skeletal homeostasis in normal bone
is performed by osteoclastic resorption of bone balanced by
osteoblastic activity. The pathophysiology of PD is characterized
by focal abnormalities in the balance of bone turnover
with increased bone resorption by the osteoclast (an increase
in osteoclast number, size, and activity)5. The chronic and
progressive nature of PD eventually results in the replacement
of normal bone with new disorganized bone that is structurally
inferior and prone to pathological fracture as in the present
case.
An estimated 70% of radiographically visible pagetic disease
sites remain clinically silent3. The majority of patients with
PD are asymptomatic and only approximately 30% experience
symptoms3,6. PD is diagnosed by exam, serum alkaline phosphatase
(AP) levels, plain radiographs, and bone scintigraphy in
the majority of patients. Alkaline phosphatase, a blood marker
of osteoblastic activity, and urinary N-telopeptide, a marker of
osteoclastic activity released during bone resorption in PD are
reflective of this increased cellular turnover. Both have been
reported to be elevated from 10-20 fold in patients with Paget’s
disease7. In the present case the AP was elevated 3-6 fold (249
IU/L).
Medications such as bisphosphonates and calcitonin are
the mainstay of treatment for PD and have been successful
in treating the disease in a variety of settings including spinal
stenosis8 and cauda equina syndrome9. The proven efficacy
of pharmacological therapy for pagetic spinal stenosis1 makes
surgical intervention a second line treatment after failure of
anti-pagetic medicines. In the present case of vertebral collapse
and instability causing myeloradiculopathy, acute operative
intervention was necessary.
PD of the spine has been reported to cause spinal stenosis
with extradural ossification and involvement of the soft tissues10,12
and invasion of the intevertebral disk13. In the case
described that presented to our institution, there was seen
to be a large bony callus that eroded both into the adjacent
soft tissues and into the intervertebral disk. Decompressive
laminectomy10 and percutaneous vertebroplasty11 have been
described in the setting of spinal PD. The present case involves
a patient with pagetic involvement of the T12 and L1 vertebral
bodies with invasion of the paraspinous tissues and vertebral
collapse causing retropulsion onto the conus. The lack of a clear
histopathological diagnosis by CT-guided transpedicular biopsy
and the local invasion seen on imaging studies merited an open
biopsy to rule out a more sinister lesion. Operative vertebrectomy,
decompression, and anterior cage placement was necessary
to alleviate impingement on the conus and to stabilize the
thoracolumbar junction. The vertebral instability and kyphotic
deformity required fusion from T10 to L3.
The etiology of PD is unknown. Genetic factors and infectious
agents have both been proposed to play a role. PD is often
seen to cluster in families and in certain geographic areas an
estimated 15% of affected individuals have at least one other
family member affected14. Pagetic osteoclasts contain nuclear
inclusions seen on electron microscopy that are thought to
represent the molecular fingerprints of the nucleocaspids of
paramyxoviruses15 suggesting a viral etiology, however an infectious
agent has yet to be isolated.
Malignant degeneration has been shown to occur in
pagetic lesions with approximately 1% progressing to osteosarcoma
(a several thousand-fold greater risk than the general
population)16,17. There is evidence to suggest an association
exists between PD and osteosarcoma that may be the result of a
single common gene on chromosome 18q or two tightly linked
genes that undergo concomitant mutation18. Careful histologic
inspection is therefore warranted in cases of PD to rule-out
sarcomatous degeneration.
Few reports exist in the literature of isolated and severe spinal
deformity secondary to PD in a young healthy person. Here
we present a focal and advanced case of PD in a 38 year-old man
causing pathologic compression fracture and cord compression
at the thoracolumbar junction. Interestingly, younger patients
with PD are more likely to have spinal involvement. A significant
difference in patients <40 and in those>40 has been observed
in the incidence of pagetic lesions in the thoracic (44% vs. 14%,
respectively) and lumbar spines (50% vs 29%, respectively)19.
In patients diagnosed with PD at age 70, subclinical pagetic
lesions are estimated to be present before the age of 30 in 64%20,
however diagnosis is rarely made this early in life.
The present case demonstrates a rare clinical occurrence
of a young healthy man with an advanced form of PD causing
vertebral collapse that required acute spinal decompression
and open biopsy. Lesions present in this younger age group
are often subclinical and represent a diagnostic and therapeutic
challenge to the orthopaedic surgeon. Early diagnosis
and treatment of PD is necessary in this age group to prevent
advanced disease presentations and to avoid neurological and
mechanical sequelae.
Darren Lebl M.D. is a PGY-2 Resident in the Harvard Combined Orthopedic Surgery Program.
Paul Glazer M.D. is a Clinical Instructor of Orthopedic Surgery at Harvard Medical School.
Address correspondence to:
Paul Glazer M.D.
Beth Israel Deaconess Medical Center
Department of Orthopedics
330 Brookline Ave.
Boston, MA 02215
References:
- Hadjipavlou AG, Gaitanis LN, Katonis PG, Lander P. Paget’s disease of the spine and its management. Eur Spine J. 2001;10:370-84.
- Roodman GD, Windle JJ. Paget disease of bone. J Clin Invest. 2005;115:200-8.
- Meunier PJ, Salson C, Mathieu L, Chapuy MC, Delmas P, Alexandre C, Charhon S. Skeletal distribution and biochemical parameters of Paget’s disease. Clin Orthop Relat Res. 1987:37-44.
- Altman RD, Bloch DA, Hochberg MC, Murphy WA. Prevalence of pelvic Paget’s disease of bone in the United States. J Bone Miner Res. 2000;15:461-5.
- Deftos LJ. Treatment of Paget’s disease--taming the wild osteoclast. N Engl J Med. 2005;353:872-5.
- Mirra JM, Brien EW, Tehranzadeh J. Paget’s disease of bone: review with emphasis on radiologic features, Part I. Skeletal Radiol. 1995;24:163-71.
- Alvarez L, Peris P, Pons F, Guanabens N, Herranz R, Monegal A, Bedini JL, Deulofeu R, Martinez de Osaba MJ, Munoz-Gomez J, Ballesta AM. Relationship between biochemical markers of bone turnover and bone scintigraphic indices in assessment of Paget’s disease activity. Arthritis Rheum. 1997;40:461-8.
- Cicuttini F, Baro G, Littlejohn G. Paget’s disease of the thoracic spine. A case report. Australas Radiol. 1990;34:177-80.
- Eulry F, Poirier JM, Perard D, Bergamasco P, Lechevalier D, Magnin J. Cauda equina syndrome with pagetic vertebral fusion. Clinical recovery under calcium-vitamin D supplementation plus clodronate after apparent failure of pamidronate and acquired resistance to etidronate. Rev Rhum Engl Ed. 1997;64:495-9.
- Hepgul K, Nicoll JA, Coakham HB. Spinal cord compression due to pagetic spinal stenosis with involvement of extradural soft tissues: a case report. Surg Neurol. 1991;35:143-6.
- Kremer MA, Fruin A, Larson TC, 3rd, Roll J, Weil RJ. Vertebroplasty in focal Paget disease of the spine. Case report. J Neurosurg. 2003;99:110-3.
- Hadjipavlou A, Shaffer N, Lander P, Srolovitz H. Pagetic spinal stenosis with extradural pagetoid ossification. A case report. Spine. 1988;13:128-30.
- Lander P, Hadjipavlou A. Intradiscal invasion of Paget’s disease of the spine. Spine. 1991;16:46-51.
- Merlotti D, Gennari L, Galli B, Martini G, Calabro A, De Paola V, Ceccarelli E, Nardi P, Avanzati A, Nuti R. Characteristics and familial aggregation of Paget’s disease of bone in Italy. J Bone Miner Res. 2005;20:1356-64.
- Rebel A, Basle M, Pouplard A, Malkani K, Filmon R, Lepatezour A. Towards a viral etiology for Paget’s disease of bone. Metab Bone Dis Relat Res. 1981;3:235-8.
- Haibach H, Farrell C, Dittrich FJ. Neoplasms arising in Paget’s disease of bone: a study of 82 cases. Am J Clin Pathol. 1985;83:594-600.
- Hansen MF, Nellissery MJ, Bhatia P. Common mechanisms of osteosarcoma and Paget’s disease. J Bone Miner Res. 1999;14 Suppl 2:39-44.
- Nellissery MJ, Padalecki SS, Brkanac Z, Singer FR, Roodman GD, Unni KK, Leach RJ, Hansen MF. Evidence for a novel osteosarcoma tumor-suppressor gene in the chromosome 18 region genetically linked with Paget disease of bone. Am J Hum Genet. 1998;63:817-24.
- Holgado S, Rotes D, Guma M, Monfort J, Olive A, Carbonell J, Tena X. Paget’s disease of bone in early adult life. Ann Rheum Dis. 2005;64:306-8.
- Renier JC, Audran M. Polyostotic Paget’s disease. A search for lesions of different durations and for new lesions. Rev Rhum Engl Ed. 1997;64:233-42.
|